Introduction
Solid, liquid and gas are the three physical states of matter. All the three states of matter have their own properties which distinguish them from each other. Substances having distinct shape and volume are termed as solids: e.g., sugar cubes, copper sulphate crystals, a wooden piece. Substances which occupy the shape of vessel, in which they are placed, are termed as liquids. 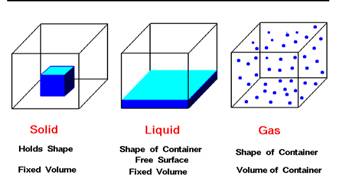 Liquids have distinct volume but do not have distinct shape: e.g., water, alcohol and benzene whereas gases do not have any distinct shape and distinct volume: e.g., Air, oxygen, and nitrogen. By providing sufficient amount of energy they are transformed from one phase to another.
Liquids have distinct volume but do not have distinct shape: e.g., water, alcohol and benzene whereas gases do not have any distinct shape and distinct volume: e.g., Air, oxygen, and nitrogen. By providing sufficient amount of energy they are transformed from one phase to another.
There are two types of interactions operating between any physical states of matter. They are:
- Intermolecular interactions- forces of attraction and repulsion binds the molecules together
- Thermal energy- energy due to random motion keeps the molecule apart.
These two forces are the basis of existence for states of matter. In case of solids, liquids and gases the intermolecular forces and thermal energy varies inversely to each other.
Thermal Energy
The total internal energy of the system or matter is the sum of various forms of energy such as potential energy, kinetic energy and nuclear energy. Internal energy arising due to potential energy is termed as chemical energy. Chemical energy keeps the electrons of atoms or molecules bound together whereas nuclear energy binds the nucleons in a nucleus. The internal energy of the system arising due to kinetic energy of the atoms or molecules is referred as thermal energy. Thus, thermal energy develops due to haphazard or random movement of atoms or molecules in a mass of matter. Thermal energy directly varies with the temperature of the matter.
Source of Thermal Energy
The Sun is the ultimate natural source of thermal energy. The nuclear fusion of hydrogen nuclei in the sun liberates high amount of thermal energy with the formation of heavy isotope of hydrogen called deuterium. This thermal energy reaches the earth in the form of radiation where it is referred as heat. Heat is the thermal energy which transfers from one system at higher temperature to another system at lower temperature. Once the system reaches to same temperature, the exchange of heat between two systems ceases and attains an equilibrium state.
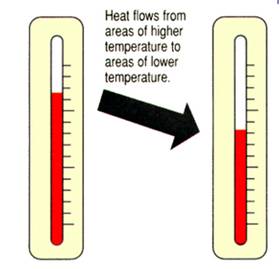 Thermal Energy and Heat
Thermal Energy and Heat
Heat and thermal energy are different from each other.
Thermal energy arises due to disorder movement of atoms or molecules while heat is absorbed or liberated during exchange of energy.
Matter posses thermal energy but it do not posses heat. Example: When we heat milk in a pan, the bottom of the vessel gets heated and this heat is transferred as thermal energy to the milk inside the pan.
Example: When we heat milk in a pan, the bottom of the vessel gets heated and this heat is transferred as thermal energy to the milk inside the pan.
Due to increase in temperature, the molecule begins to move in various directions at greater speed.
Thus, the whole of the milk inside the pan gets heated and begins to boil.
How thermal energy is different from heat energy?
Try to answer. Still need help? Want to know more about it? Click here to schedule live help from a certified tutor!
About eAge Tutoring:
eAgeTutor.com is the premium online tutoring provider. Using materials developed by highly qualified educators and leading content developers, a team of top-notch software experts, and a group of passionate educators, eAgeTutor works to ensure the success and satisfaction of all of its students.
Contact us today to learn more about our guaranteed results and discuss how we can help make the dreams of the student in your life come true!
Reference Links:
- http://en.wikipedia.org/wiki/Solids
- http://itl.chem.ufl.edu/2045/lectures/lec_g.html
- http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/inteng.html
- http://www.youtube.com/watch?v=03XCnYLWGac
- http://sjesci.wikispaces.com/file/view/StatesOfMatter.gif/146334781/StatesOfMatter.gif
- http://www.g9toengineering.com/resources/heatflow.gif