English Guide: Practical Tips for Better English Communication
 The last two decades have been hugely transformational as digital technologies have disrupted every walk of life. This will only continue in the new decade, as machine learning, deep learning and artificial intelligence (AI) begin to play an integral part in our workplaces. As a result, workplaces will require very different skillsets than they needed until now. If you are a youngster looking for rapid career development in this new decade, then...
The last two decades have been hugely transformational as digital technologies have disrupted every walk of life. This will only continue in the new decade, as machine learning, deep learning and artificial intelligence (AI) begin to play an integral part in our workplaces. As a result, workplaces will require very different skillsets than they needed until now. If you are a youngster looking for rapid career development in this new decade, then...
 A career in sales can be very exciting and lucrative for people who do well in it. Sales brings in revenues for any organisation, and revenues help companies move forward. As long as one is performing well and continues to bring in sales, one’s job is fairly secure because in times of adversity businesses slash costs, while trying to keep revenues high. Yet, success in a sales career isn’t guaranteed for everyone. It takes certain personality...
A career in sales can be very exciting and lucrative for people who do well in it. Sales brings in revenues for any organisation, and revenues help companies move forward. As long as one is performing well and continues to bring in sales, one’s job is fairly secure because in times of adversity businesses slash costs, while trying to keep revenues high. Yet, success in a sales career isn’t guaranteed for everyone. It takes certain personality...
 In a recent article, we wrote about the difference between improve and improvise and how to use these two similar terms appropriately. Continuing this series on using similar-sounding English words correctly, let us consider the use of the words “affect” and “effect”.
Both terms mean “impact” or “result” of something, but their usage varies depending on the context in which they are being used.
Affect
The word “affect” is generally used in the...
In a recent article, we wrote about the difference between improve and improvise and how to use these two similar terms appropriately. Continuing this series on using similar-sounding English words correctly, let us consider the use of the words “affect” and “effect”.
Both terms mean “impact” or “result” of something, but their usage varies depending on the context in which they are being used.
Affect
The word “affect” is generally used in the...
 English language has plenty of words that seem very similar but mean very different. It is quite easy to ignore the subtle difference in the words themselves and consider them to mean the same thing. This results in incorrect usage of the words when writing or speaking in English, which may confuse the listener or create a negative impression about your English Communication skills. Let’s focus on two words, improve and improvise; the former is a...
English language has plenty of words that seem very similar but mean very different. It is quite easy to ignore the subtle difference in the words themselves and consider them to mean the same thing. This results in incorrect usage of the words when writing or speaking in English, which may confuse the listener or create a negative impression about your English Communication skills. Let’s focus on two words, improve and improvise; the former is a...
 Someone has rightly said, “In the mundane, nothing is sacred. In sacredness, nothing is mundane.”
Mundane is boring. There comes a time when you feel saturated with the routine tasks you are doing and you feel like doing different and challenging things. Your ardent desire to take up additional work and prove your worth overwhelms you. But this needs to be communicated effectively to your boss. Your boss should be aware that you are geared up...
Someone has rightly said, “In the mundane, nothing is sacred. In sacredness, nothing is mundane.”
Mundane is boring. There comes a time when you feel saturated with the routine tasks you are doing and you feel like doing different and challenging things. Your ardent desire to take up additional work and prove your worth overwhelms you. But this needs to be communicated effectively to your boss. Your boss should be aware that you are geared up...
 Gratitude is of vital importance in human life. We need to thank people who have, directly or indirectly, explicitly or implicitly, made a positive change in our life. ‘Thank You’ is the least that we can say. So never shy away from that.
In professional life too, gratefulness is of utmost importance, especially when someone has helped you climb up the career ladder.
It’s your promotion party and you have to give a thank you speech – this...
Gratitude is of vital importance in human life. We need to thank people who have, directly or indirectly, explicitly or implicitly, made a positive change in our life. ‘Thank You’ is the least that we can say. So never shy away from that.
In professional life too, gratefulness is of utmost importance, especially when someone has helped you climb up the career ladder.
It’s your promotion party and you have to give a thank you speech – this...
 We all have encountered situations wherein there were unavoidable or unforeseen circumstances because of which we had to extend our leave. For example, sickness, family issues, marriage etc. Now here comes the uncomfortable question – how to ask the boss for a leave extension. Quite tricky, huh?
When you are already on leave and then you have to request for extension of leave, it should be done in such a way that it is granted without much...
We all have encountered situations wherein there were unavoidable or unforeseen circumstances because of which we had to extend our leave. For example, sickness, family issues, marriage etc. Now here comes the uncomfortable question – how to ask the boss for a leave extension. Quite tricky, huh?
When you are already on leave and then you have to request for extension of leave, it should be done in such a way that it is granted without much...
 A few months back, a video of a job prospect venting out his frustrations on having to speak in English at job interviews, went viral. The protagonist of the video, a youngster, who could speak English fluently was angry that he was always asked to speak in English, and he let out his frustration with a series of expletives in Hindi. The fact that the video went viral and was shared by a large audience in the country, especially among...
A few months back, a video of a job prospect venting out his frustrations on having to speak in English at job interviews, went viral. The protagonist of the video, a youngster, who could speak English fluently was angry that he was always asked to speak in English, and he let out his frustration with a series of expletives in Hindi. The fact that the video went viral and was shared by a large audience in the country, especially among...
 Man is a social animal. We cannot survive in isolation. Also, we all crave for love and attention, no denying that. We are constantly expanding our social network. Be it personal or professional life, networking is of utmost importance to us: Social networking, for getting love and affection, and professional networking for career enhancement.
In this competitive world, we cannot lounge around waiting for opportunities to knock at our door. We...
Man is a social animal. We cannot survive in isolation. Also, we all crave for love and attention, no denying that. We are constantly expanding our social network. Be it personal or professional life, networking is of utmost importance to us: Social networking, for getting love and affection, and professional networking for career enhancement.
In this competitive world, we cannot lounge around waiting for opportunities to knock at our door. We...
 Someone has rightly said, “Good conversation starts with good listening”. Our teachers have often told us that we wouldn’t learn anything new from talking but we will definitely learn something new from listening. How true!
There’s a lot of buzz about spoken English these days, but people tend to forget that English listening is also an integral and inseparable part of English communication, which cannot be overlooked.No wonder then the...
Someone has rightly said, “Good conversation starts with good listening”. Our teachers have often told us that we wouldn’t learn anything new from talking but we will definitely learn something new from listening. How true!
There’s a lot of buzz about spoken English these days, but people tend to forget that English listening is also an integral and inseparable part of English communication, which cannot be overlooked.No wonder then the...
 India is undergoing a digital revolution. The country has embarked on the ‘Digital India’ movement that has been officially promoted by the Indian government. It is therefore not surprising to see a jump in career opportunities in the field of digital marketing, especially search engine marketing (SEM). This field attracts a lot of freshers and young job aspirants. Having good English skills will enable a person to take advantage of the growing...
India is undergoing a digital revolution. The country has embarked on the ‘Digital India’ movement that has been officially promoted by the Indian government. It is therefore not surprising to see a jump in career opportunities in the field of digital marketing, especially search engine marketing (SEM). This field attracts a lot of freshers and young job aspirants. Having good English skills will enable a person to take advantage of the growing...
 Good manners will open doors that the best education cannot.”
Etiquette is the science of living. It embraces everything.People all over the world are judged, liked, disliked, remembered or looked down with contempt based on their manners. Good manners speak a lot about your personality.
It is imperative that you display your best behaviour when you are in a public place especially when eating out. When you walk into a restaurant you don’t want to...
Good manners will open doors that the best education cannot.”
Etiquette is the science of living. It embraces everything.People all over the world are judged, liked, disliked, remembered or looked down with contempt based on their manners. Good manners speak a lot about your personality.
It is imperative that you display your best behaviour when you are in a public place especially when eating out. When you walk into a restaurant you don’t want to...
 Good teamwork is essential not only for the growth of any organisation but also for the personal growth of individuals in the organisation. An essential component of achieving good team bonding is when team members express positive thoughts about fellow team members to them directly. Sincere compliments and praise go a long way in making a person feel good and feel motivated to perform better for the team and the organisation.
There are a...
Good teamwork is essential not only for the growth of any organisation but also for the personal growth of individuals in the organisation. An essential component of achieving good team bonding is when team members express positive thoughts about fellow team members to them directly. Sincere compliments and praise go a long way in making a person feel good and feel motivated to perform better for the team and the organisation.
There are a...
 At the outset, let us state that we hope you never have to apply for a compassionate leave. However, the reality is that most of us, at some stage of our life, end up having to request for such leaves. This article is written to specifically help you in case of such an eventuality.
Before we get into the mechanics of applying for compassionate leave, let us first understand what is compassionate leave. It is leave granted to an employee upon death...
At the outset, let us state that we hope you never have to apply for a compassionate leave. However, the reality is that most of us, at some stage of our life, end up having to request for such leaves. This article is written to specifically help you in case of such an eventuality.
Before we get into the mechanics of applying for compassionate leave, let us first understand what is compassionate leave. It is leave granted to an employee upon death...

Electrolysis

Introduction to Electrolysis
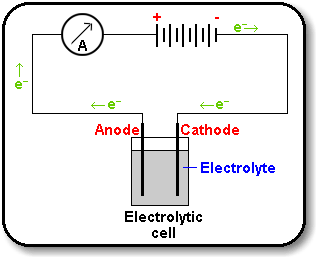 What is Electrolysis
What is Electrolysis
Electrolysis is the process that people use to generate hydrogen (and oxygen) from water. The word "lysis" means to dissolve or break apart, so the word "electrolysis" literally means to break something apart (in this case water) using electricity.
Electrolysis is very simple -- all you have to do is arrange for electricity to pass through some water between electrodes that are placed in the water, as the diagram above shows. It’s as simple as that! Michael Faraday first formulated the principle of electrolysis in 1820.
The key process of electrolysis involves the interchange of atoms and ions that occurs through the removal or addition of electrons from the external circuit. The desired products of electrolysis are in a different physical state from the electrolyte and can be removed through various physical processes. For example, when brine is subjected to electrolysis to produce hydrogen and chlorine, the products are gaseous. These gaseous products bubble from the electrolyte and are easily collected.
Components of Electrolysis
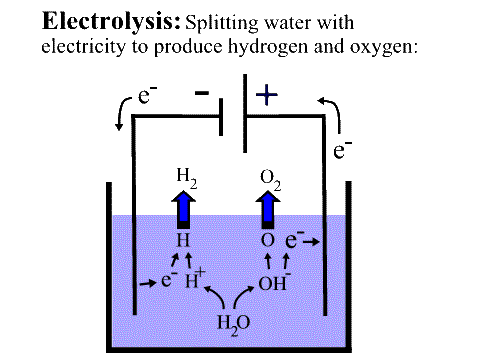 The main components that are needed to achieve electrolysis are:
The main components that are needed to achieve electrolysis are:
- An electrolyte: an electrolyte is a substance containing free ions. These ions are the carriers of the electric current in the electrolyte. If the ions are not mobile, as in solid salt, then electrolysis cannot occur.
- A direct current (DC) supply: a DC supply provides the energy necessary to create or discharge the ions in the electrolyte. The electric current is carried by electrons in the external circuit.
- Two electrodes: these function as an electrical conductor which provides the physical interface between the electrical circuit providing the energy and the electrolyte.
At the cathode (the negative electrode), the battery creates a negative charge. Consequently, there is electrical pressure to push electrons into the water at this end. At the anode (the positive electrode), there is a positive charge, so that electrode would like to absorb electrons. But the water isn't a very good conductor. Instead, in order for there to be a flow of charge all the way around the circuit, water molecules near the cathode are split up into a positively charged hydrogen ion, which is symbolized as H+ in the diagram above, and a negatively charged "hydroxide" ion, symbolized as OH-:
H2O -> H+ + OH- .
A positively charged hydrogen ion, or H+, is just the hydrogen atom without its electron, i.e. the nucleus of the hydrogen atom, which is just a single proton.
You might have expected that H2O would break up into an H and an OH (the same atoms but with neutral charges), but this doesn't happen because the oxygen atom more strongly attracts the electron from the H -- it steals it. (Officially, we say the oxygen atom is more "electronegative" than hydrogen). This theft allows the resulting hydroxide ion to have a completely filled outer shell, making it more stable.
But because of this transfer, the H+, which is just a naked proton, is now free to pick up an electron (symbolized as e-) from the cathode, which is trying hard to donate electrons. When H+ picks up an electron, it will become a regular, neutral hydrogen atom:
H+ + e- -> H
When this hydrogen atom meets another hydrogen atom, they will form a hydrogen gas molecule:
H + H -> H2,
And this molecule will bubble to the surface -- we have hydrogen gas!
Meanwhile, the positive anode has caused the negatively charged hydroxide ion (OH-) to travel across the container to the anode. When it gets to the anode, the anode removes the extra electron that the hydroxide stole from the hydrogen atom earlier, and the hydroxide ion then recombines with three other hydroxide molecules to form 1 molecule of oxygen and 2 molecules of water:
4 OH- -> O2 + 2 H2O + 4e-
The oxygen molecule is very stable, and bubbles to the surface.
In this way, a closed circuit is created, involving negatively charged particles -- electrons in the wire, hydroxide ions in the water. The energy delivered by the battery is stored as the production of hydrogen.
***
Want to know more about Electrolysis? Click here to schedule a live session with an eAge eTutor!
About eAge Tutoring:
eAgeTutor.com is the premium online tutoring provider. Using materials developed by highly qualified educators and leading content developers, a team of top-notch software experts, and a group of passionate educators, eAgeTutor works to ensure the success and satisfaction of all of its students.
Contact us today to learn more about our guaranteed results and discuss how we can help make the dreams of the student in your life come true!




