English Guide: Practical Tips for Better English Communication
 The last two decades have been hugely transformational as digital technologies have disrupted every walk of life. This will only continue in the new decade, as machine learning, deep learning and artificial intelligence (AI) begin to play an integral part in our workplaces. As a result, workplaces will require very different skillsets than they needed until now. If you are a youngster looking for rapid career development in this new decade, then...
The last two decades have been hugely transformational as digital technologies have disrupted every walk of life. This will only continue in the new decade, as machine learning, deep learning and artificial intelligence (AI) begin to play an integral part in our workplaces. As a result, workplaces will require very different skillsets than they needed until now. If you are a youngster looking for rapid career development in this new decade, then...
 A career in sales can be very exciting and lucrative for people who do well in it. Sales brings in revenues for any organisation, and revenues help companies move forward. As long as one is performing well and continues to bring in sales, one’s job is fairly secure because in times of adversity businesses slash costs, while trying to keep revenues high. Yet, success in a sales career isn’t guaranteed for everyone. It takes certain personality...
A career in sales can be very exciting and lucrative for people who do well in it. Sales brings in revenues for any organisation, and revenues help companies move forward. As long as one is performing well and continues to bring in sales, one’s job is fairly secure because in times of adversity businesses slash costs, while trying to keep revenues high. Yet, success in a sales career isn’t guaranteed for everyone. It takes certain personality...
 In a recent article, we wrote about the difference between improve and improvise and how to use these two similar terms appropriately. Continuing this series on using similar-sounding English words correctly, let us consider the use of the words “affect” and “effect”.
Both terms mean “impact” or “result” of something, but their usage varies depending on the context in which they are being used.
Affect
The word “affect” is generally used in the...
In a recent article, we wrote about the difference between improve and improvise and how to use these two similar terms appropriately. Continuing this series on using similar-sounding English words correctly, let us consider the use of the words “affect” and “effect”.
Both terms mean “impact” or “result” of something, but their usage varies depending on the context in which they are being used.
Affect
The word “affect” is generally used in the...
 English language has plenty of words that seem very similar but mean very different. It is quite easy to ignore the subtle difference in the words themselves and consider them to mean the same thing. This results in incorrect usage of the words when writing or speaking in English, which may confuse the listener or create a negative impression about your English Communication skills. Let’s focus on two words, improve and improvise; the former is a...
English language has plenty of words that seem very similar but mean very different. It is quite easy to ignore the subtle difference in the words themselves and consider them to mean the same thing. This results in incorrect usage of the words when writing or speaking in English, which may confuse the listener or create a negative impression about your English Communication skills. Let’s focus on two words, improve and improvise; the former is a...
 Someone has rightly said, “In the mundane, nothing is sacred. In sacredness, nothing is mundane.”
Mundane is boring. There comes a time when you feel saturated with the routine tasks you are doing and you feel like doing different and challenging things. Your ardent desire to take up additional work and prove your worth overwhelms you. But this needs to be communicated effectively to your boss. Your boss should be aware that you are geared up...
Someone has rightly said, “In the mundane, nothing is sacred. In sacredness, nothing is mundane.”
Mundane is boring. There comes a time when you feel saturated with the routine tasks you are doing and you feel like doing different and challenging things. Your ardent desire to take up additional work and prove your worth overwhelms you. But this needs to be communicated effectively to your boss. Your boss should be aware that you are geared up...
 Gratitude is of vital importance in human life. We need to thank people who have, directly or indirectly, explicitly or implicitly, made a positive change in our life. ‘Thank You’ is the least that we can say. So never shy away from that.
In professional life too, gratefulness is of utmost importance, especially when someone has helped you climb up the career ladder.
It’s your promotion party and you have to give a thank you speech – this...
Gratitude is of vital importance in human life. We need to thank people who have, directly or indirectly, explicitly or implicitly, made a positive change in our life. ‘Thank You’ is the least that we can say. So never shy away from that.
In professional life too, gratefulness is of utmost importance, especially when someone has helped you climb up the career ladder.
It’s your promotion party and you have to give a thank you speech – this...
 We all have encountered situations wherein there were unavoidable or unforeseen circumstances because of which we had to extend our leave. For example, sickness, family issues, marriage etc. Now here comes the uncomfortable question – how to ask the boss for a leave extension. Quite tricky, huh?
When you are already on leave and then you have to request for extension of leave, it should be done in such a way that it is granted without much...
We all have encountered situations wherein there were unavoidable or unforeseen circumstances because of which we had to extend our leave. For example, sickness, family issues, marriage etc. Now here comes the uncomfortable question – how to ask the boss for a leave extension. Quite tricky, huh?
When you are already on leave and then you have to request for extension of leave, it should be done in such a way that it is granted without much...
 A few months back, a video of a job prospect venting out his frustrations on having to speak in English at job interviews, went viral. The protagonist of the video, a youngster, who could speak English fluently was angry that he was always asked to speak in English, and he let out his frustration with a series of expletives in Hindi. The fact that the video went viral and was shared by a large audience in the country, especially among...
A few months back, a video of a job prospect venting out his frustrations on having to speak in English at job interviews, went viral. The protagonist of the video, a youngster, who could speak English fluently was angry that he was always asked to speak in English, and he let out his frustration with a series of expletives in Hindi. The fact that the video went viral and was shared by a large audience in the country, especially among...
 Man is a social animal. We cannot survive in isolation. Also, we all crave for love and attention, no denying that. We are constantly expanding our social network. Be it personal or professional life, networking is of utmost importance to us: Social networking, for getting love and affection, and professional networking for career enhancement.
In this competitive world, we cannot lounge around waiting for opportunities to knock at our door. We...
Man is a social animal. We cannot survive in isolation. Also, we all crave for love and attention, no denying that. We are constantly expanding our social network. Be it personal or professional life, networking is of utmost importance to us: Social networking, for getting love and affection, and professional networking for career enhancement.
In this competitive world, we cannot lounge around waiting for opportunities to knock at our door. We...
 Someone has rightly said, “Good conversation starts with good listening”. Our teachers have often told us that we wouldn’t learn anything new from talking but we will definitely learn something new from listening. How true!
There’s a lot of buzz about spoken English these days, but people tend to forget that English listening is also an integral and inseparable part of English communication, which cannot be overlooked.No wonder then the...
Someone has rightly said, “Good conversation starts with good listening”. Our teachers have often told us that we wouldn’t learn anything new from talking but we will definitely learn something new from listening. How true!
There’s a lot of buzz about spoken English these days, but people tend to forget that English listening is also an integral and inseparable part of English communication, which cannot be overlooked.No wonder then the...
 India is undergoing a digital revolution. The country has embarked on the ‘Digital India’ movement that has been officially promoted by the Indian government. It is therefore not surprising to see a jump in career opportunities in the field of digital marketing, especially search engine marketing (SEM). This field attracts a lot of freshers and young job aspirants. Having good English skills will enable a person to take advantage of the growing...
India is undergoing a digital revolution. The country has embarked on the ‘Digital India’ movement that has been officially promoted by the Indian government. It is therefore not surprising to see a jump in career opportunities in the field of digital marketing, especially search engine marketing (SEM). This field attracts a lot of freshers and young job aspirants. Having good English skills will enable a person to take advantage of the growing...
 Good manners will open doors that the best education cannot.”
Etiquette is the science of living. It embraces everything.People all over the world are judged, liked, disliked, remembered or looked down with contempt based on their manners. Good manners speak a lot about your personality.
It is imperative that you display your best behaviour when you are in a public place especially when eating out. When you walk into a restaurant you don’t want to...
Good manners will open doors that the best education cannot.”
Etiquette is the science of living. It embraces everything.People all over the world are judged, liked, disliked, remembered or looked down with contempt based on their manners. Good manners speak a lot about your personality.
It is imperative that you display your best behaviour when you are in a public place especially when eating out. When you walk into a restaurant you don’t want to...
 Good teamwork is essential not only for the growth of any organisation but also for the personal growth of individuals in the organisation. An essential component of achieving good team bonding is when team members express positive thoughts about fellow team members to them directly. Sincere compliments and praise go a long way in making a person feel good and feel motivated to perform better for the team and the organisation.
There are a...
Good teamwork is essential not only for the growth of any organisation but also for the personal growth of individuals in the organisation. An essential component of achieving good team bonding is when team members express positive thoughts about fellow team members to them directly. Sincere compliments and praise go a long way in making a person feel good and feel motivated to perform better for the team and the organisation.
There are a...
 At the outset, let us state that we hope you never have to apply for a compassionate leave. However, the reality is that most of us, at some stage of our life, end up having to request for such leaves. This article is written to specifically help you in case of such an eventuality.
Before we get into the mechanics of applying for compassionate leave, let us first understand what is compassionate leave. It is leave granted to an employee upon death...
At the outset, let us state that we hope you never have to apply for a compassionate leave. However, the reality is that most of us, at some stage of our life, end up having to request for such leaves. This article is written to specifically help you in case of such an eventuality.
Before we get into the mechanics of applying for compassionate leave, let us first understand what is compassionate leave. It is leave granted to an employee upon death...

Equipartition of Energy

Introduction to the Equipartition of Energy
The equipartition of energy theorem states: each degree of freedom contributes on average ½ kT per molecule to the stored energy of a gas. k is Boltzman’s constant, i.e., k = R/ N = 1.38 x 10-23 T/K. R = the universal gas constant and N = Avogadro number
Thus for a monatomic molecule of an ideal gas which has three degrees of freedom per molecule kinetic energy = 3/2 kT (for one molecule of gas):
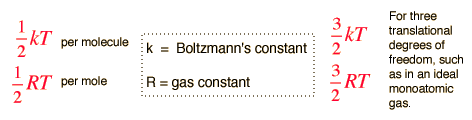
The equipartition result is as follows: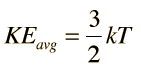
Equipartition energy for diatomic gases
Similarly, a molecule of diatomic gas has 5 degrees of freedom per molecule. Therefore, kinetic energy per molecule is 5/2 kT and mean kinetic energy per mole is 5/2 kT × N (where N is number of molecules in one mole; N is called the Avogadro number). Further the number of molecules per cc at NTP in all gases is the same and is independent of the nature of gas. This number is called Loschmidt’s number.
We say that the different ‘modes’ (translational, rotational or vibrational modes) are excited, i.e. they ‘come into play’, and contribute to the heat capacity of a gas in different temperature ranges. At low temperatures, only translational modes are excited – i.e., cV is 3/2 R per mole, and the rotational and vibrational modes are said to be ‘frozen out’.
The measured value of cV around room temperature (~300 K) is 5/2 R: both translational (3 modes) and vibrational modes (2 modes) contribute.
Total Translational energy
The total translational energy of all the molecules of a gas is
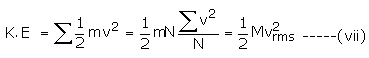
The average kinetic energy of a molecule is
![]()
![]()
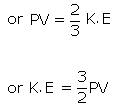
Experimentally, the contributions of the degrees of freedom to energy can be observed in measurements of cV.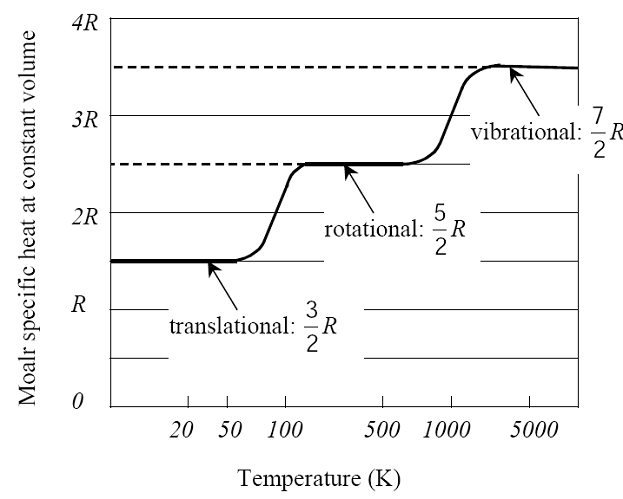
Want to know more about the equipartition of energy? Click here to schedule a live session with an eAge eTutor!
About eAge online Tutoring
eAgeTutor.com is the premium online tutoring provider. Using materials developed by highly qualified educators and leading content developers, a team of top-notch software experts, and a group of passionate educators, eAgeTutor works to ensure the success and satisfaction of all of its students.
Contact us today to learn more about our guaranteed results and discuss how we can help make the dreams of the student in your life come true!




